Change of state
BUCUN BENGKEL : Most substance change
their physical state with the additional or removal of heat.
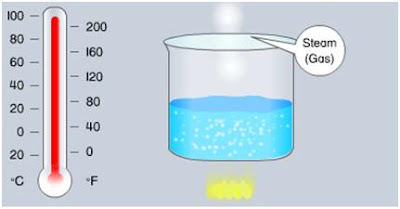
For instance, water ice is solid at temperature below 32°F (0°C). If heat is added to the ice, it will melt and become water (liquid) – Latent Heat. When water is heated continuously, it boil at 212°F (100°C) (Temperature increased) – Sensible heat. Further additional of heat will cause the water to turn into vapor (gas) – Latent Heat. This process is also reversible. When the same amount of heat is removed the steam will change to water. When further heat is removed from the water it will change into ice. The compression type refrigeration cycle uses this principle.
For instance, water ice is solid at temperature below 32°F (0°C). If heat is added to the ice, it will melt and become water (liquid) – Latent Heat. When water is heated continuously, it boil at 212°F (100°C) (Temperature increased) – Sensible heat. Further additional of heat will cause the water to turn into vapor (gas) – Latent Heat. This process is also reversible. When the same amount of heat is removed the steam will change to water. When further heat is removed from the water it will change into ice. The compression type refrigeration cycle uses this principle.
The Water ice is solid at temperature below 32°F (0°C)
Water ice will melt and become water (liquid) if heat is added
Water ice turn into vapor (gas)
Source : Panasonic